Oncology Imaging CRO Services
We are an imaging research and core laboratory that provides comprehensive imaging CRO (iCRO) services and technology solutions throughout the clinical trial process.
IE is the largest oncology-focused iCRO globally, and is widely recognized for successfully conducting the industry’s most pivotal imaging trials in oncology. IE’s iCRO experience spans hundreds of successful trials across all phases of development, including some of the most high-profile registration trials and regulatory approvals across the globe.
Connecting Imaging to the Cure to Better Serve You.
Imaging Endpoints partners with sponsors in their effort to develop new therapeutics and diagnostics that will improve lives and ultimately cure the type of cancer each trial targets.
Our project teams consist of project managers, physicians and medical / scientific affairs (SA) experts, data managers, technologists and compliance experts with vast experience in oncology trials.
Our experienced physicians and SA experts focus in oncology and go further than any imaging CRO to customize your analysis to provide the optimal chance to
prove efficacy.
IE’s Operations team aligns with SA to execute customized image acquisition and analysis with excellence and provides real-time data that makes your work easy.
Our Imaging Clinical Trial Management System (iCTMS) and reporting processes enable us to interact with sites and provide real-time information you need. Our services are tailored to match your project’s unique objectives while providing the agility and passion needed in a clinical trial partner.

8 Offices in 6 countries across America, Europe and Asia

100 Dedicated Physician Readers, >40 In-House Radiologists

100s of Successful Clinical Trials, Most Impactful Regulatory Approvals Globally

Thousands of Clinical Trial Sites across the World
Setting New Standards in Quality
- IE’s Imaging Quality Management System (iQMS) is designed to exceed industry standards.
- All inspections in 2018/2019/2020/2021/2022 by FDA (USA), EMA (Europe), CFDA (China), PMDA (Japan) and MFDS (Korea) resulted in zero observations clearly demonstrating our leadership in quality, compliance and regulatory approvals.
Services Description:
Study Management and Partnership
- Expert project managers and a unique project team designed to partner with your team throughout the trial
- Comprehensive real-time reporting to make your job easy
- Development and maintenance of a Project Management Plan and Timeline
- Imaging CTMS (iCTMS) for detailed and real-time subject/scan tracking, electronic query resolution, and dashboard reporting
- Frequent and effective communication in your preferred formats
Study Startup with Customization
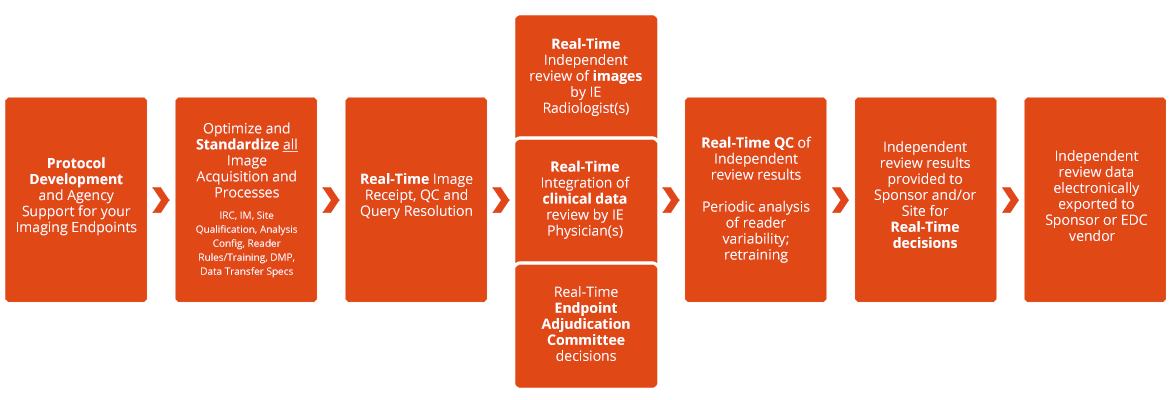
- Protocol Development
- Participation at kick-off and investigator meetings
- Setup electronic TMF and iCTMS
- Creation of Imaging Review Charter in compliance with FDA Guidance; includes expert criteria customization to your unique trial for optimal chance to prove efficacy
- Imaging site assessment and qualification
- Site Imaging Manual preparation and site training
- Digital imaging transfer setup – global, real-time image transfer and receipt 24/7/365. Transfer software eliminates PHI/PII and minimizes queries.
- Standardization with phantoms
- Reader selection, reader training, and training verification
- Data management plan, data validation plan, and data transfer specifications
Study Conduct, Exquisite Services
- Scan receipt, QC, and query resolution in real time
- We work relentlessly to resolve queries until they are resolved.
- Expert reads in real time
- Rapid reads and reporting that meets your specific needs and budget
- Experienced radiologists
- >100 in-house and expert board certified and subspecialty trained radiologists and other physician specialists (oncologists, surgeons, etc.)
- Excellence in Analysis
- State-of-the-art cloud-based analysis software available globally
- Close reader oversight to ensure quality reads and minimize reader variability, with guaranteed results
- Excellence in data management
- Real-time data monitoring and verification of reads
- Data exports on a scheduled or as-needed basis
Study Closure and Reporting in Partnership
-
- eTMF maintenance and closure
- eTMF maintained according to current regulations and real-time access
- Perform routine reconciliation
- Perform all tasks required to clean and lock the eTMF
- Imaging data maintenance and closure
- Maintain image archive according to current regulations and real-time access
- Perform routine reconciliation
- Final delivery of TMF and quality-confirmed images with viewing software
- Secure Storage
- Secure storage including remote backup in ready access cloud-based environment. Geographical segmentation globally available.
- eTMF maintenance and closure
Our Promise: Exquisite Services
Imaging Endpoints works closely with sponsors to provide outstanding service that is customized to meet their needs. In addition to routine daily, weekly, and monthly communications, our Project Managers as well as our Scientific Affairs experts and top management quickly return calls, and we provide mobile numbers for issues that arise outside of business hours. We are readily available to address your questions and ensure your project needs are always met.
Meet Our Enthusiastic Clients!
Imaging Endpoints works with some of the largest biopharma companies as well as many medium and small companies. We are happy to share references and case studies with you. Please contact us to learn more.

